Overview
The Health Research Ethics Board of Alberta Cancer Committee (HREBA-CC) provides ethical review and oversight for all cancer and cancer-related research involving humans (adult and pediatric), their information and/or samples. This includes both research that involves or does not involve health information as defined by the Alberta Health Information Act (HIA).
Information on how to submit and manage an ethics application is available in the PAGES menus to the right.
Please note:
- All new protocols submitted to the HREBA-CC must be through IRISS.
- All existing open files have been migrated to IRISS, and are no longer in paper format. If you are unclear how to proceed, please email cancer@hreba.ca.
Meeting Dates and Deadlines
The HREBA Cancer Committee meets twice a month.
There are two deadlines for each meeting:
- applications and amendments must be must be received by the Cancer Committee office two weeks before the Cancer Committee meeting. This is the submission deadline. Check here for specific dates: 2024 Cancer Committee Dates and Deadlines
- renewals must be received one week before the meeting date.
Minimal risk applications including chart reviews, image reviews and laboratory based studies are processed through the delegated review pathway.
Multiple Sites in Alberta
For clinical trials conducted by multiple principal investigators at sites within AHS Cancer Control there is a single review process to reduce duplication of ethics review and the associated administrative burden. This process requires increased communication among researchers, research teams, Clinical Research Unit staff, and the HREBA-CC administrative staff.
Standard Operating Procedures
HREBA has adopted the Standard Operating Procedures (SOPs) developed by the Network of Networks (N2) and the Canadian Association of Research Ethics Boards (CAREB). These SOPS are compliant with applicable Canadian and US regulatory and ethics guidance criteria. They facilitate the distribution, adoption and maintenance of a single set of standards for REBs in Canada.
Use of SOPs also ensures that the ethics board is constituted appropriately and protects the rights and safety of study participants.
You may find them listed here with links to the pdf documents.
Fees
Research studies funded by industry or other for-profit organizations are charged an administration fee for the initial ethics review. Details are found here.
Contact Information
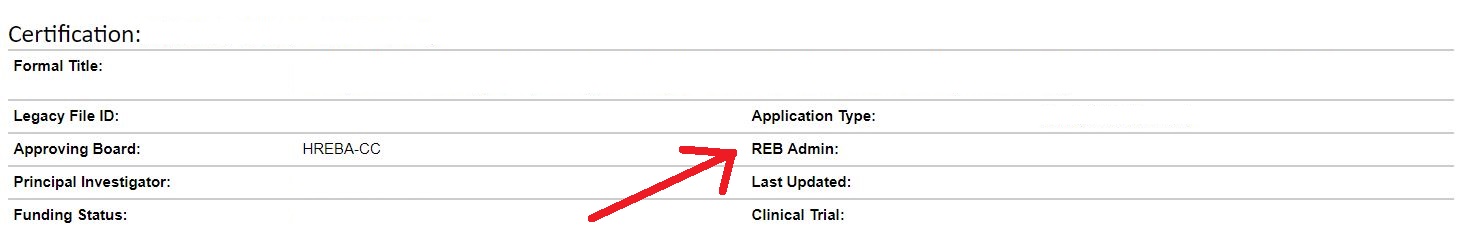
If you have questions about a specific file, please contact the HREBA coordinator listed as the “REB Admin”. Contact information can be found here.
If you have a general question, please e-mail cancer@hreba.ca.

